
What is being done now to increase compliance of sterilization and reduce Healthcare Associated Infections? For more information regarding proper sterilization guidelines visit: The financial benefit of using these prevention practices is estimated to be $25.0 billion to $31.5 billion in medical cost savings, but most importantly would be the reduction of preventable deaths and healthcare-related infections. Studies have shown that with the proper education and training, health care workers increased compliance and adoption of best practices to prevent Heath Acquired Infections. Prevention of Healthcare Associated Infections
Sterilization non-compliant rates in 2013 It has been reported, that 1 in 25 hospital patients has at least one healthcare-associated infection.Īccording to Joint Commission, (a non-profit organization that accredits and certifies healthcare organizations nationwide), in 2013 the non-compliance of hospitals, ambulatory and office-based surgery facilities to reduce the risk of infections associated with medical equipment, devices, and supplies was one of the top five non-compliant requirements.
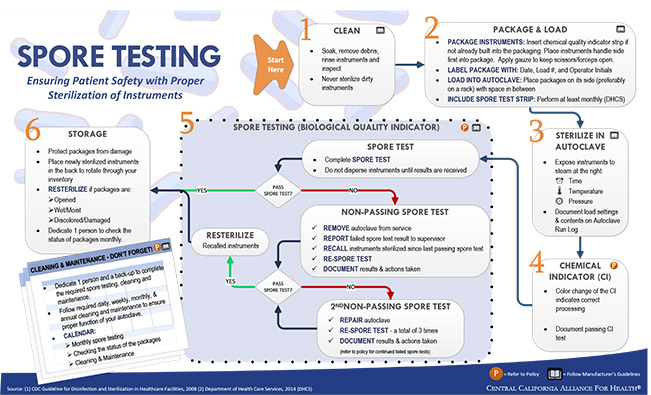
Healthcare Associated Infections are among the leading causes of preventable deaths and the most common complication of hospital care in the United States. What is deeply concerning is the astounding rates of sterilization non-compliance. These same standards should apply regardless whether the patient care is being provided, in an acute care hospital, ambulatory surgical center, outpatient facility, dental or physician’s office. Knowing and understanding the principles and various sterilization methods, in addition to consistent monitoring, helps to ensure effective sterilization, patient safety, and cost-effectiveness. The federal guidelines for infection control and sterilization include the requirement that weekly spore tests should be performed and the results filed.

Hospitals and Ambulatory Centers are required to be in compliance with the Federal requirements outlined in the Medicare Conditions of Participation to receive Medicare/Medicaid payment. Yes, the proper sterilization of medical devices, surgical instruments, supplies and equipment utilized in patient care and surgery is a critical aspect of health care that directly impacts patient safety.


 0 kommentar(er)
0 kommentar(er)
